S1.5 Ideal Gases
1.5.1: Assumptions of Ideal Gases:
An ideal gas is a theoretical gas composed of many randomly moving point particles that interact only through elastic collisions and obey the gas laws perfectly under all conditions of temperature and pressure.

Boyle's Law:
Boyle’s law states that pressure and volume are inversely proportional:
Graph One highlights the relationship between pressure and volume. Whereas graph two shows a proportional relationship between pressure and the reciprocal of volume.

P1 x V1 = P2 x V2
P - Pressure (Kpa)
V - volume (dm³)
1.5.2 : Real gases vs Ideal gases
The main difference between an ideal gas and a real gas lies in how they respond to changes in pressure. An ideal gas follows Boyle’s Law perfectly, showing an inverse relationship between pressure and volume; as pressure increases, volume decreases smoothly and predictably. However, real gases deviate from this behavior, especially at high pressures. This deviation occurs because real gas particles occupy space and experience intermolecular forces, factors ignored in the ideal gas model
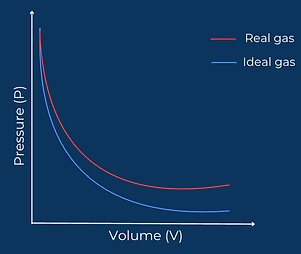
NOTE : To maintain ideal gas condition we need: High temperature & Low pressure
1.5.3 : Molar Volume
Referring to structure 1.4 – Avogadro’s law states that at constant temperature and pressure, equal volumes of gases contain equal numbers of moles (particles). Hence the molar volume (Vm) of an ideal gas is constantly specified at a specific temperature and pressure.
n = Vm
Where Vm = 22.7 dm³/mol at STP (Standard temperature and pressure: 100kPa, 273K)
1.5.4 : The Ideal Gas Laws
Charles Law:
V1 V1
T1 T1
Where V is volume in (dm³) and T is temperature in (Celsius) of two gases.
Volume is directly proportional to temperature at constant pressure. As the temperature increases,the volume increases.
=
Gay-Lussacs Law:
P1 P1
T1 T1
Where P is Pressure and T is temperature in (Celsius) of two gases.
Pressure is directly proportional to temperature at constant volume. As the temperature increases, pressure increases.
=
Combined Gas-Law
P1 x V1 P1x V1
Combines Boyle’s, Charles’s, and Gay-Lussac’s laws. Used when pressure, volume, andtemperature all change, assuming constant moles.
=
T1
T1
The Ideal Gas Law
PV = nRT
Relates pressure, volume, number of moles, and temperature of a gas. Apply to ideal gases under standard conditions.
Where:
P - Pressure (Pa)
V - Volume (M³)
n - Number of Moles (mol)
R - constant (8.314 Pa·m³/mol·K)
T - temperature (K)
